A Pocket Guide to Healthcare Compliances
Healthcare is one of the dominant sectors that is being bombarded with digital technologies in an effort to modernize its infrastructural landscape and services.
Taking institutional spending as a yardstick, healthcare tops US government spending by a good stretch in correspondence with other sectors. In 2017, the US allocated $3.5 trillion to healthcare. As a share of the country’s GDP, this amounted to 17.7 percent. Healthcare, in the US, is not a territory confined to legacy businesses with truckloads of capital to invest and expand but open to all for participation.
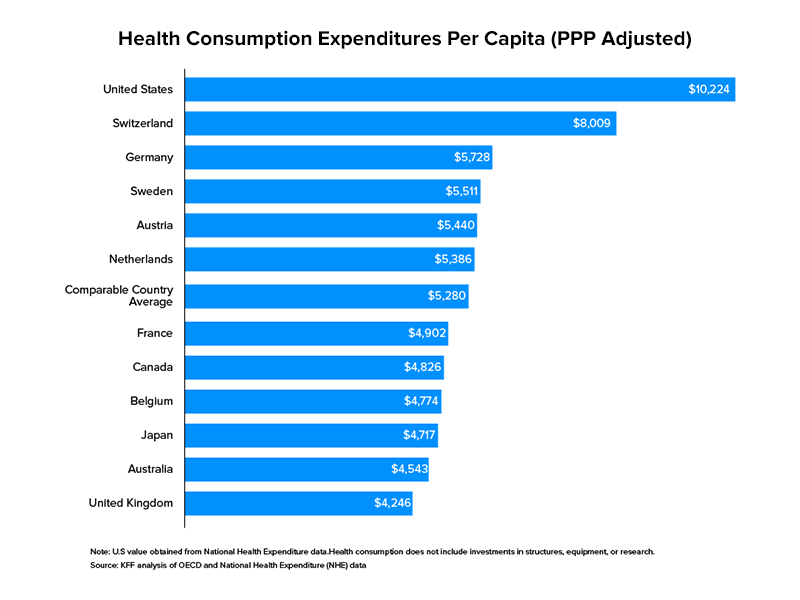
Digital health is a developing prospect of this industry that encompasses business models like telehealth, remote monitoring through smartphone apps and doorstep delivery of prescription drugs. In 2019 alone, more than $17 billion were raised by startups advocating digital healthcare adoption in over 1,700 deals within the States. As expected out of this ongoing shift, mobile healthcare development is taking centre stage.
Developing a product that claims to impact/improve personal hygiene is a tough nut to crack, not because of the healthcare software development services challenges involved, but because of the fiduciary guidelines.
Appinventiv is at the leading edge of this change pulsing the heartbeat of the industry thanks to our partners. As a healthcare app development company, we have been there and done that, which was necessary to make ground-breaking ideas fruition to life. As your confidant, today we’ll be discussing the common regulations and compliance that you must keep in mind when developing an mHealth application.
What Is Healthcare Regulation Compliance?
Regulatory compliance in Healthcare is about a medical association’s adherence to laws, guidelines, regulations, and specifications applicable to its business processes. Infringement of regulatory compliance regularly brings about legal punishment including federal fines. It is the continuous cycle of meeting or surpassing the lawful, moral, and professional norms relevant to a specific healthcare association or supplier.
Currently, healthcare companies like hospitals and healthcare providers have dedicated team members who specially focus on regulatory compliance. The reason and essential advantage of regulatory requirements for hospitals are to improve patient care.
What Is The Purpose Of Compliance In Health Care?
Healthcare compliance is a fundamental segment of each medical provider. It is the way of following rules, guidelines, laws, and regulations that relate with healthcare services practice.
Compliance in healthcare covers a wide assortment of practices and observes inner and external rules. However, most medical services compliance issues identify with patient security, the protection of patient data, and billing rehearses.
The U.S. Branch of Health and Human Services (HHS) has made country wide standards known as the Health Insurance Portability and Accountability Act (HIPAA), and the center feature of medical compliance is upholding this legislation.
Why is Healthcare Compliance Important?
Compliance keeps tasks to run smoothly and ensure everybody follows legitimate procedures and understands assumptions.
Yet, compliance in medical services accompanies significantly higher stakes than in different industries. On the other hand if medical practitioners like doctors and nurses don’t follow legitimate procedure, then they can end up harming a patient or another staff member.
Ultimately, healthcare compliance is about providing safe, high-quality patient care. Complying with industry standards and regulations helps healthcare organizations continue to improve the quality of care.
At last, medical care compliance is about giving protected, quality, safe, patient care. Complying to industry guidelines and regulations helps healthcare organizations to keep on improving the quality of care.
Healthcare organizations are held to strict guidelines, laws, and regulations from the government. Violations of these laws can bring in lawsuits, hefty fines, or even loss of licenses.
What is High Risk For Compliance Issues?
A regulatory framework serves as a model utilized for sanctioning guidelines. Such structures might be created inside explicit areas of interest, like the healthcare industry. Governments frequently depend on the utilization of such frameworks to create and enact guidelines, rules, and laws. Regulatory frameworks are commonly first developed considering the end goal in mind.
Let’s look into the main question that is bugging everyone, that is what are compliance issues in healthcare?
HIPAA & Data Breaches
Value-based Compensation Arrangements
Anti-Kickback & Physician-Hospital Issues
Due Diligence Processes
Telehealth Provider Qualifications
Recovery Audit Contractors
Tax-Exempt Hospitals & Compliance Requirements
Healthcare Regulations and Compliance in US
Digital health has elaborative strictures pointing out the dos and don’ts for Health Information Technology (HIT), mobile health, personalized prescriptions, wearable technology and telehealth. Mobile apps are one of the most common ways of healthcare delivery, be it through an offline-online service or software as a service. If that is the case, entrepreneurs must chalk out the following questions and find objective answers to them:

The above questionnaire would demarcate if your mobile app needs fiduciary clearance or not. Provided your answers were mostly yes, there are three federal bodies that’ll look into the matter of your mHealth product:
- Food and Drug Administration (FDA)
- Federal Trade Commission (FTC)
- Office of Civil Rights (OCR)
Below we take a look at key federal areas of function and the scope of granular fact-checking that a healthcare software development company must advise its clients on.
1. Health Insurance Portability and Accountability Act (HIPAA)
This act is enforced by the Office for Civil Rights (OCR) within the US Department of Health and Human Services. This medical compliant HIPAA security rule safeguards the privacy and security concerns of eligible health-related data and in particular cases, deems reporting data breaches mandatory. Failing to abide by a particular HIPAA security rule could result in penalties starting from a minimum payment of $100 and go up as high as $1.5 million on a per-incident violation basis.
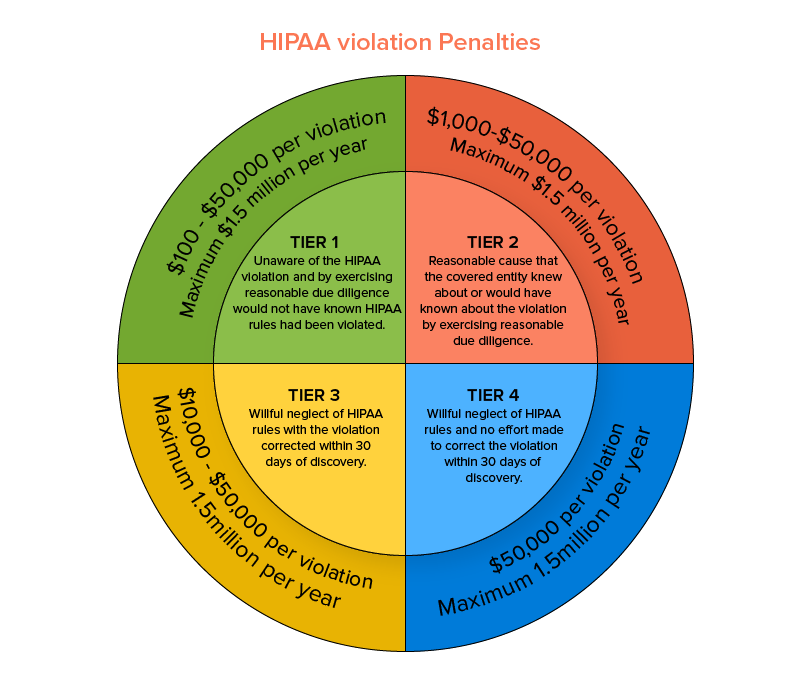
Now that you have a palpable idea of the seriousness with which to approach the healthcare regulations requirement, we should proceed and look into the types of medical apps that should comply with the traditional policies of HIPAA. Three 3 factors govern the eligibility criteria laid out for an app to be distinguished as medical in operational terms:
I. The nature of the entity that uses the application.
Entity refers to the customer who’d be using the app. There is a set of predefined medical practitioners that are covered by the constitution of HIPAA such as doctors, physicians, organizations such as hospitals and health insurance providers. If they are the direct beneficiaries of the app, then the list of healthcare regulations and hospital regulatory compliance by HIPAA will have to be followed word for word. On the other hand, if the app simply curates and shares hygiene tips or wellness knowledge with the customer, that’ll be exempt from HIPAA’s constitution.
II. The nature of data the application produces, preserves and shares further.
Data is critical to the functioning needs of online businesses. Federal authorities push for laws that negate security concerns like data breaches and ensure the presence of robust encryption infrastructure. In essence, the collected data should not and must not lead malicious actors to people via their personal information such as an address, social security number etc. If the app is going to deal in the usage of such personal pin-points, then HIPAA rules will apply.
III. The underlying software that powers the application.
Best of breed healthcare mobile app development must concentrate on innovating a secure phone app. HIPAA shares the details of the Protected Health Information (PHI) and directs software vendors to grind a safety net around it. Its directives have a profound checklist of audits and internal controls to be installed for PHI.
2. Federal Trade Commission Act (FTC Act)
This act imposes regulatory protocols to deal with unfair claims and malpractices in businesses, also relating to issues of privacy and general data security challenges. Unfounded claims about the usage of an app are covered by this law. FTC’s Health Breach Notification Rule mandates select businesses to report data breaches such as personal health records.
3. Federal Food, Drug, and Cosmetic Act (FD&C Act)
The Food and Drug Administration is entrusted to implement this act. Their prime aim is to ensure that medical devices, mobile applications included, qualify standard guidelines and are therefore safe to be consumed en masse. It’s paramount we mention that not all healthcare apps fall under this jurisdiction but a select few. These are the ones that if fail to deliver on claims pose serious consequences to consumer health.
Additional Regulation For Digital Health
While the above acts were incisively targeted healthcare applications, there are others which weren’t instituted for the reason but adjusted to include the same. In this section we’ll gauge an overview of such state-backed norms that mHealth entrepreneurs must adhere to.
1. Food and Drug Administration (FDA)
The Food and Drug Administration is a US government-backed agency that constitutes a key component of the US Department of Health and Human Services. Healthcare app developers must heed to such a set of well-defined guidelines while engineering apps to procure an FDA clearance. For the digital health sector, the FDA categorizes mobile apps into “medical” slabs based on the following two postulates:
- The app is utilized as an accessory alongside or together with an already regulated medical device.
- The app morphs the mobile platform into a regulated mobile device
Based on the aforementioned first-level classification, the sub-sector of the app is defined based on its relation to the following emerging digital technologies to inch closer to an FDA approval.
I. Software as a Medical Device (SaMD)
SaMD is defined as a model where software is employed for medical purposes without being associated with a hardware medical instrument/device. The model is highly flexible and can be applied to a range of platforms from virtual networks to medical devices.
The International Medical Device Regulators Forum (IMDRF) is a global coalition that advocates systematic governance of medical devices. In 2013 it formulated the Software as a Medical Device Working Group (SaMDWG) to introduce actionable guidance to support the advancement of digital technologies in this segment. Headed by the FDA itself, the group has documented a plethora of frameworks on:
- Definitions
- Risk categorization
- Quality Management System
- Clinical evaluation
Going through their catalogues will help you identify if you’ll procure a SaMD approval or not.
II. Wireless Medical Devices
It refers to medical devices equipped with the capability of carrying out a wireless transmission of information slash data to facilitate healthcare services. Such toolkits deploy Radio Frequencies for communication which can be transmitted over WiFi, Bluetooth or a smartphone. A common example, one that you might have found in corporate offices is the Radio Frequency Identification (RFID) devices.
III. Telemedicine
Health IT is bifurcated into Telemedicine and Telehealth in order to simplify the process of obtaining FDA approval. Telehealth is designated as the use of telecommunications to promote and support healthcare-related functions – one that has come all the more into the limelight since COVID-19 outbreak. A custom healthcare software development company could develop applications that use:
- Live (asynchronous) Videoconferencing
- Store-and-forward (asynchronous) Videoconferencing
- Remote Patient Monitoring (RPM)
- Mobile Health (mHealth)
IV. Health IT
In this case, we’d better quote the definition provided by the federal Gov. Office of the National Coordinator for Health Information Technology, “hardware, software, integrated technologies or related licenses, intellectual property, upgrades, or packaged solutions sold as services that are designed for or support the use by health care entities or patients for the electronic creation, maintenance, access, or exchange of health information.”
V. Medical Device Data Systems (MDDS)
Hardware/software products that can be used to channel data, preserve/store information, convert data from one format to another or simply display scientific/medical data fall under the MDDS category. The objective of such devices is not to contribute additional characteristics or enhance the data set but simply display it.
VI. Medical Device Interoperability (MDI)
There is arguably no other digital health subdomain where the concept of a secure phone app bodes perfectly well than MDI. Medical Device Interoperability refers to the cross-platform/technological exchange of information between multiple devices. Unlike MDDS, where the primary motive is presentation, MDI applications can display, store, and analyze data. As a result of the to and fro communication, they can also be used to control other products.
VII. Device Software Functions
When talking about this category, clearance is given only to those software apps that are qualified to be “devices” under the FDA guidelines. Software devices that have minimal to no risk for consumption may not require an official FDA approval and the organization is explicit when it says in such cases it
“will exercise enforcement discretion and will not expect manufacturers to submit premarket review applications or to register and list their software with the FDA.”
VIII. Cybersecurity
Cybersecurity in and of itself is not a mode of classification for an mHealth app. Yet, the FDA wants to establish a clear cut Memorandum of Understanding (MOU) so that it could weigh the data security challenges the app poses against the benefits to the users.
IX. Artificial Intelligence/Machine Learning
The impact of AI on healthcare is disproportionately gargantuan against other technologies. Yet , advancement in this sector has been rather recent due to which the FDA has had to make adjustments to its regulatory frameworks. As per its latest guidelines, the FDA will work hand in hand with manufacturers to continuously assess the software starting from its pre-market development stages and culminating at the post-market performance stage. The framework applies specifically to SaMD.
2. HL7 Standards
Health Level Seven International, simply referred to as HL7 is a non-profit organization constituted in 1983 that develops industry benchmarks for the exchange, integration, sharing and retrieval of electronic health information that enables procedural medical practice. In addition to that, HL7 standards play a rudimentary role in managing health service, staying put on course for a seamless healthcare delivery and evaluating results.
How do they do that?
HL7 standards define the packaging of information to be interoperable between two healthcare apps, dictating the workflow for language, data formats and its structure so as to easily integrate into the systems. By doing so, they bring down investments in technological infrastructure and benefit the patients in turn making healthcare more affordable. Complying to the drafted rules so to gain an HL7 approval carries dual benefits for regulating healthcare industry. Frist, the healthcare app is universally accepted and ready for deployment worldwide. And second, the cost of application development is reduced.
3. The HITECH Act
The Health Information Technology for Economic and Clinical Health Act was introduced during the regime of President Barack Obama in 2009. The purpose of the HITECH Act was to promote the enterprise adoption of Health Information Technology through Electronic Health Records (EHRs). The administration also tightened the loose ends around the HIPAA Act of 1996 following which it became mandatory for healthcare businesses to inform customers if and when their credentials were compromised.
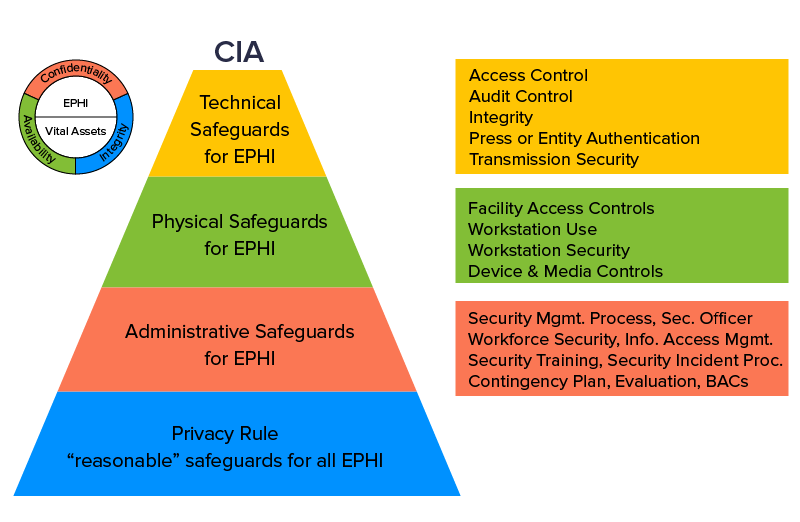
The immediate effect of the HITECH Act was that information sharing between two distinct entities became easier to handle thanks to EHRs. The act also ensured that hard-to-breach security infrastructure was installed in cohesion with the privacy and security must-haves of the HIPAA Act. All the regulatory requirements to obtain a HITECH approval were baked into HIPAA via the Final Omnibus Rule, that resulted in both the acts becoming stacked under a single legislation.
4. BYOD
The Bring Your Own Device (BYOD) is a conceptual practice where healthcare employers allow medical staff to use personal devices such as but not limited to smartphones, and tablets for official duties. Things could go downhill in an instant if your mHealth solution is not customized for BYOD security protocols. For instance, imagine a scenario where an employee loses his/her smartphone with the device having access to critical Protected Health Information (PHI).
This is where a strongly thought out Mobile Device Management strategy comes into play. Provided the app developers had built a remote wipe capability into the mHealth solution, you can erase the data associated with the lost device. Similar functionalities within the BYOD umbrella include securing client applications such as emails and browsers. Taking note of such minutiae during the initial SDLC stages could help startups procure a BYOD approval eventually.
5. GDPR
The General Data Protection Regulation was created by the European Union (EU) and applies to smartphone applications that collect and process customer data of European Union citizens. With talks of similar iterations of this act in works beyond the EU borders, it is considered a safety measure for app developers to create mHealth solutions in accordance with the same. Privacy protection is the essence of GDPR through which the federal authorities have attempted (with success) to hand over some control of personal data to the layman. It also keeps business practices pertaining to private data management out in the open.

Provisions of the GDPR require mobile apps to request permission, in other words active user consent, prior to collecting or processing their data. The app should make it easy for the user to share their accord via a checkbox or any other button to click and register the action. Additionally, such checkboxes should not be pre-ticked so as to psychologically impact the user’s choice which must stay unhindered. The Terms & Conditions page must have its own “I Agree” button. GDPR has largely democratized personal data control as even after giving their consent, should the users choose to, they can revoke all rights granted to the app and disengage. Activating a GDPR approval from the authorities would be relatively easy, provided the mobile app development company engineers a solution in view of this discussion.
Final Thoughts
The national healthcare spending is expected to reach $5.7 trillion by the year 2026 thanks to digital technologies such as mHealth, telemedicine, sensors and wearable tech and remote monitoring tools. Such healthcare trends are indicative of an uphill march of never before seen medical solutions that incorporate technology for an instant outreach to the public. They are also expected to make healthcare affordable for all as a basic right to be had and not a service to be availed. While you focus on the business side of the venture, let Appinventiv– one of the top healthcare app development agency in USA be on stand by mode as your technological guide.
We’d be waiting to hear your thoughts.

strategies your digital product..





